SPINEVISION
SpineVision, headquartered in Paris, France, is a spinal technology specialist company that dedicates itself to intensive research, development and marketing of implants and instruments for complicated spinal surgeries. Based on technology developed by SpineVision, XentiQ was entrusted with the product development of an all-digital, yet disposable surgical tool for spine treatments. This tool had to adhere to a necessary set of stringent specifications, yet has the flexibility for mass production. To ensure complete success, XentiQ had to be involved in the entire development process of this medical instrument.
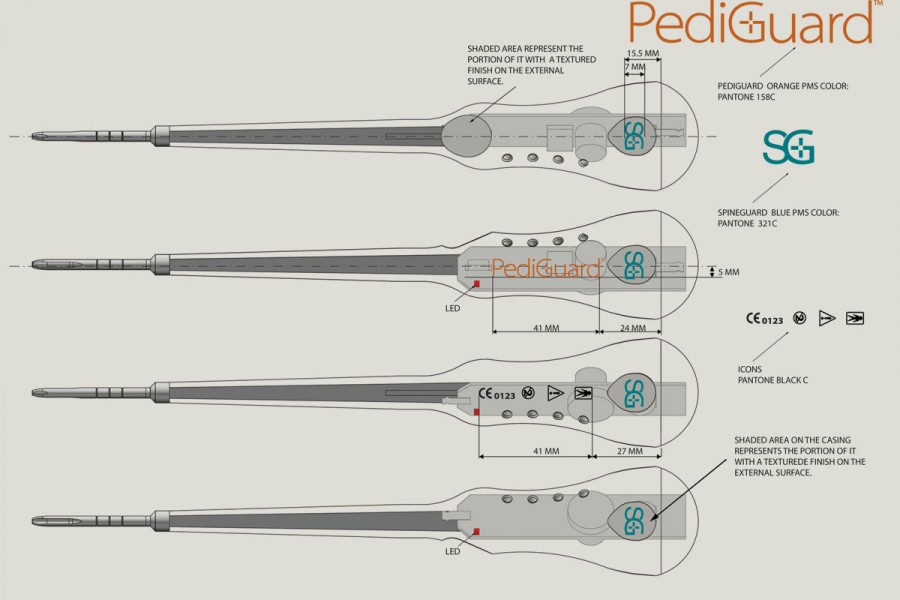
This device would be able to offer surgeons a tool to accurately detect changes in tissue type when performing pedicle screw fixation for spinal surgery. This is especially important as a reported 14% of screws are misplaced during insertion, which can cause permanent damage to the spinal cord. XentiQ had to work within strict design documentation, tests and production requirements when developing the necessary designs.
In addition to the proposed product design, XentiQ was also involved in the detailed packaging, manufacturing tests and quality-control plans necessary for the successful mass production of Pediguard.
Although commissioned by a French company, PediGuard was designed by XentiQ and manufactured entirely in Singapore. PediGuard is approved by the US Food and Drug Administration (FDA).
PARTNER WITH THE RIGHT COMPANY